There are many theories on the nature of light. These conflict in some places and bring forth the idea of wave-particle duality. Wave-particle duality is the notion that light behaves like a wave as well as like a particle.
Video:
Click/tap here to load video
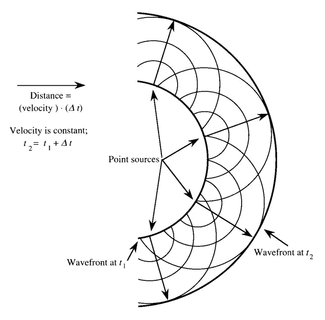
Huygen’s principle
- Proposed by Christiaan Huygens in 1678
- In summary it states that every point on a wave is a source of secondary wavelets expanding from that point. The new wave front , called the envelope of the wavelets, is tangential to the wave fronts of each of these wavelets
- The envelope of a wave studied in three-dimensions will be a sphere whereas a 2D view will show it as a circle
Credits: AP Physics B
Pros and cons
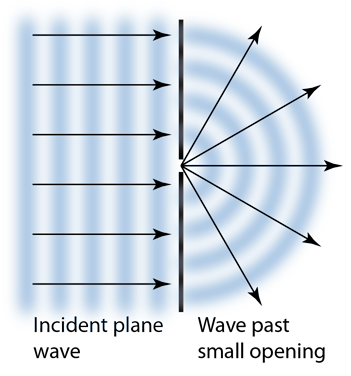
- It explains diffraction Huygens’ Principle allows us to easily explain the behaviour of waves as they diffract (bend around corners and spread as they pass through gaps) as seen in the case of a diffraction grating – the points on the wave which are between the gap produce wavelets which spread out and interfere on the screen – if light was only particles, it would only pass through the gap in a straight line
Credits: HyperPhysics
- It explains refraction The points on the wavefront which are at the boundary between the two media will produce new wavelets which travel in a direction different from that of the incident wave
- It introduces the back propagation issue It has a limitation of not being able to explain why the light does not propagate backwards – the secondary wavelets each act as a wave and they are theorized to move out from their origin point in all directions so why doesn’t light from a flashlight reach the person holding it without reflection occurring?
Newton’s corpuscular theory of light
- Newton theorized that light was made up of particles (corpuscles) rather than waves
- Newton passed a beam of white light through two prisms which were oriented in such a way that the white light was split into the visible spectrum when it passed through the first prism and these components recombined into the white light as they passed through the second prism. This was termed the ‘crucial experiment’
- Newton concluded that the white light was just the combination of coloured particles
- Newton also introduced the idea of a ‘colour spectrum’
- Despite the colours on the spectrum being continuous (there is not a fixed boundary between each colours) Newton separated them into the seven ROYGBIV categories
- He demonstrated that there are unqiue angles of refraction for each of the colours
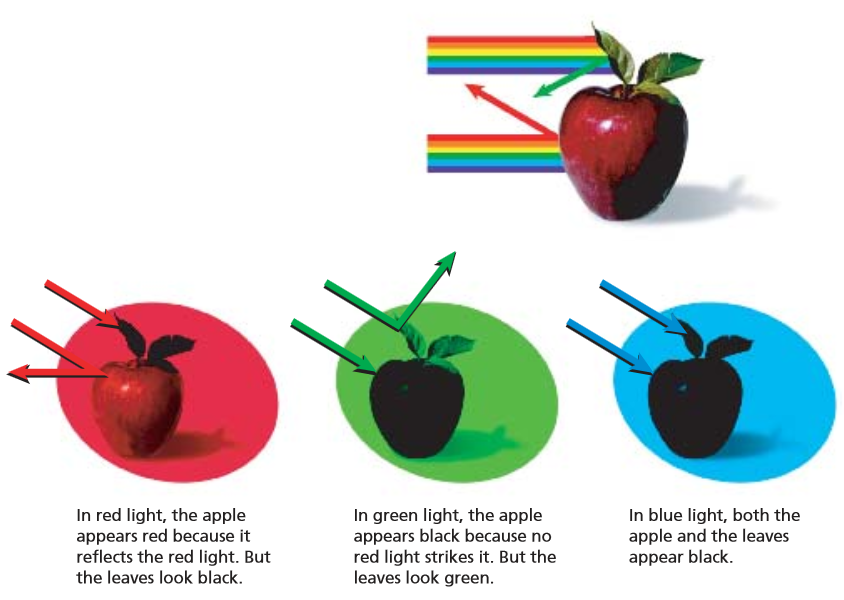
- He also concluded that the colour we see coming from an object was not a property of the object itself but rather a property of the light – an apple appears red only when the light incident upon it contains certain wavelengths on the spectrum (the ones corresponding to red) because the apple absorbs all other wavelengths
Credits: people highline edu
Pros and cons
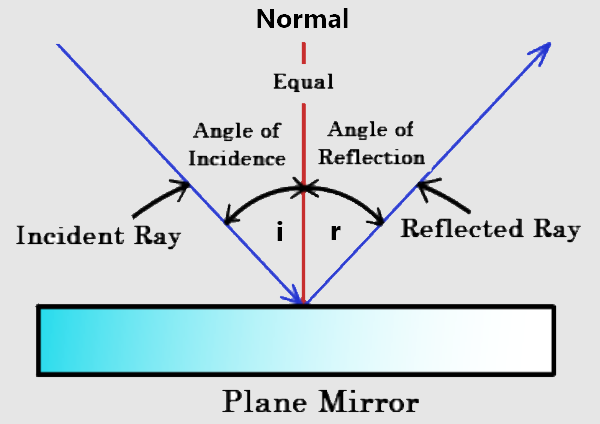
- It explains the law of reflection The theory easily explained why the angle of incidence is equal to the angle of reflection since the light is treated as a stream of particles which can bounce off a surface
Credits: Free Exam Academy
- It better explains diffuse reflection (reflection of light off of a rough surface) as incident particles will bounce off at various angles thus scattering the light
- Newton claimed that diffraction was a new type of refraction
- His corpuscular theory thus did not sufficiently explain diffraction
- Huygens’ theory better explained diffraction
Einstein’s theory of light
- Einstein explained what we now call the photoelectric effect
- The photoelectric effect is a phenomenon where photons (light particles) incident on the surface of a metal will displace electrons which can then be detected as a current
- Some of the energy of the photon is used to dislodge the electron and the remainder becomes the kinetic energy of the electron
Why it’s better than the others
- The wave theories of light failed to explain why if the light doesn’t pass a certain threshold frequency then the electron is not dislodged – regardless of how long the metal’s surface is exposed to the light
- Wave theory also cannot explain why the intensity of the light affects the rate of electron ejection rather than the amount of kinetic energy the electron has