The following describes how the various gas laws can be explained by the Kinetic Theory.
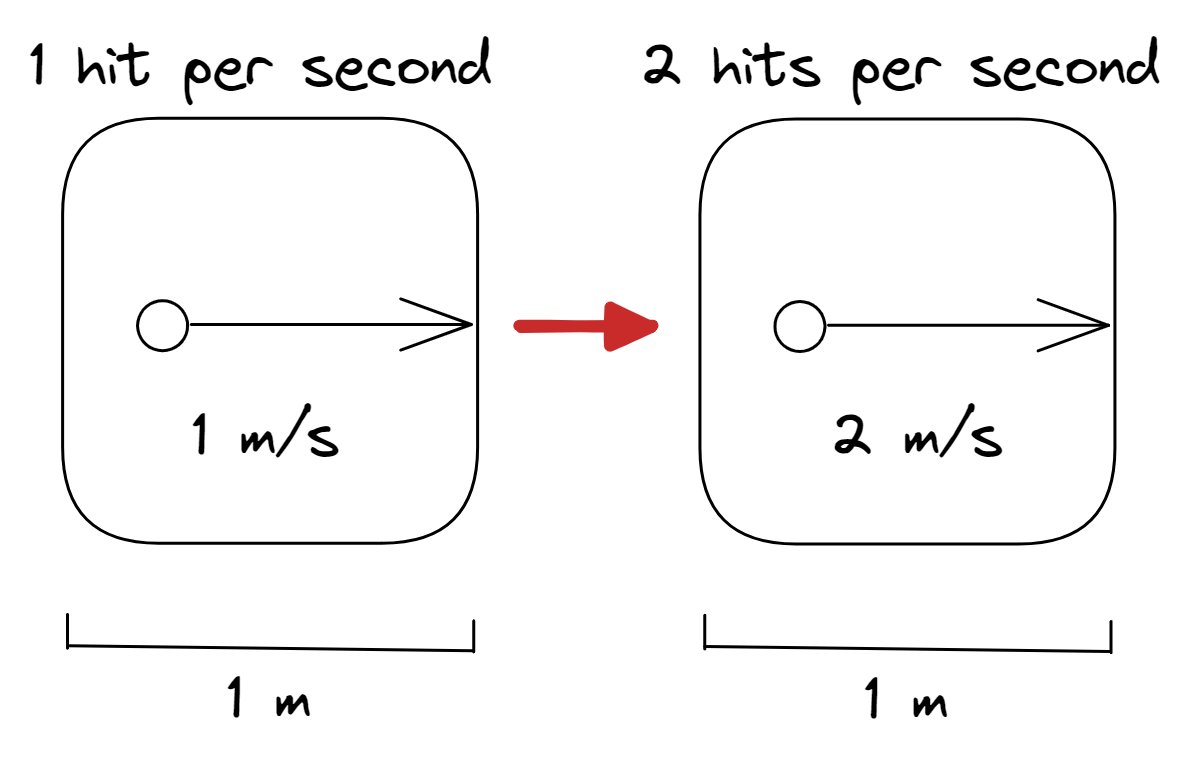
Pressure law
- Pressure is described as the frequency and strength of collisions by the gas particles with the walls of the container
- For a single gas particle trapped in a box which cannot expand (constant volume), the greater the temperature of the gas, the more kinetic energy the particle has
- The greater the kinetic energy, the greater the speed the particle has
- Greater speed implies a greater strength of collision (greater pressure)
- Greater speed also means the particle will bump into the walls of the container more often (greater pressure)
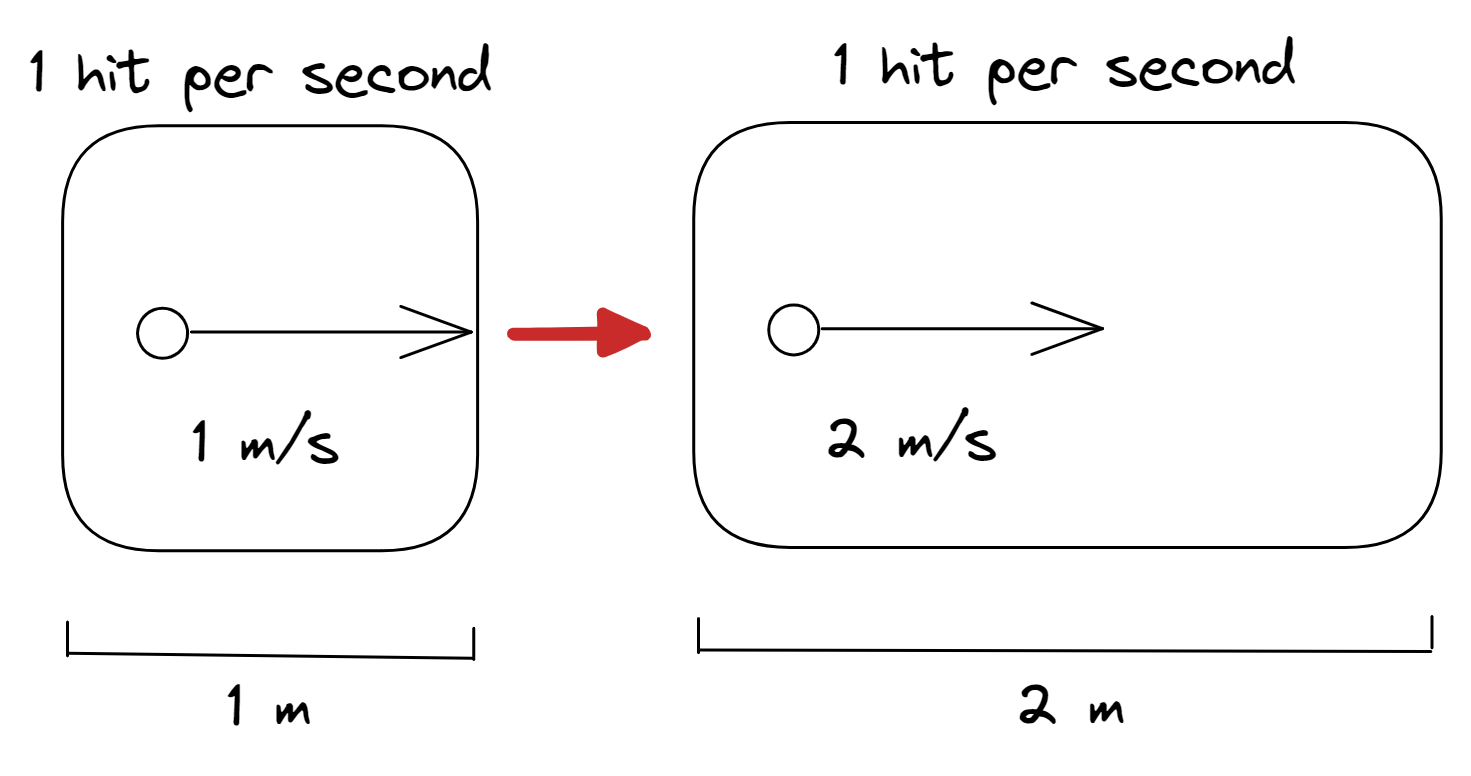
Charles’ law
- Volume is the space that a gas takes up
- When the temperature of the gas is increased, the particles increase in their kinetic energy
- Their speeds increase and they will hit the walls of the container more often
- Thus in order for there to be a constant pressure (rate at which the particles hit the walls), the walls of the container must be further apart (volume increases)
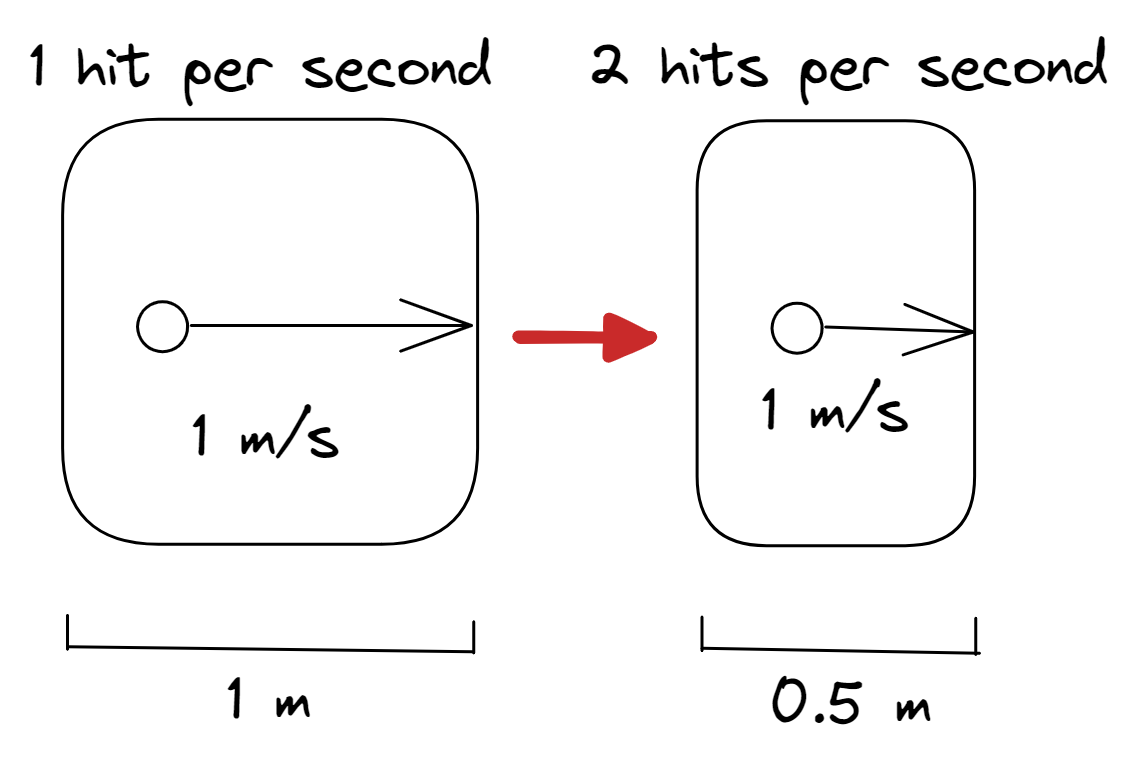
Boyle’s law
- Reducing the size of the container of the gas (volume decreases), the rate at which the particles collide with the sides of the container increases (pressure increases) at constant temperature (constant particle speed)
- Increasing the size of the container would reduce the number of collisions per unit time